

More than 90% of patients had normal hemostasis at the time of surgery or procedure. The surgeon or interventionalist assessed hemostasis, the secondary endpoint of RE-VERSE AD. The time from administration of the first vial of idarucizumab was 1.4 to 1.9 hours for surgery and 1.2 hours for catheter-based procedures. Orthopaedic (n = 45), abdominal (n = 49), and vascular (n = 34) composed the majority of surgeries. The majority (72%) of patients underwent surgery 28% had a catheter-based procedure. Levy noted, "There was rapid total reversal of dabigatran after administration of idarucizumab." More than 95% of patients achieved maximum reversal of dabigatran within 4 hours. "This reflects the real-world patient population."Īt baseline, patients undergoing surgery had an average dabigatran level of 80 to 90 ng/mL, with individuals in the catheter group having average levels of about 100 ng/mL. Most patients had multiple comorbidities, with about one-third having heart failure," Levy commented.
All patients had impaired creatinine clearance, which is important because dabigatran is principally cleared through the kidneys. "RE-VERSE AD had a complex, critical ill patient population. Diabetes, heart failure, coronary artery disease, and previous stroke or transient ischemic attack were common baseline characteristics. In addition, more than 90% of those undergoing surgery had AF all patients in the drainage and catheter groups had AF.

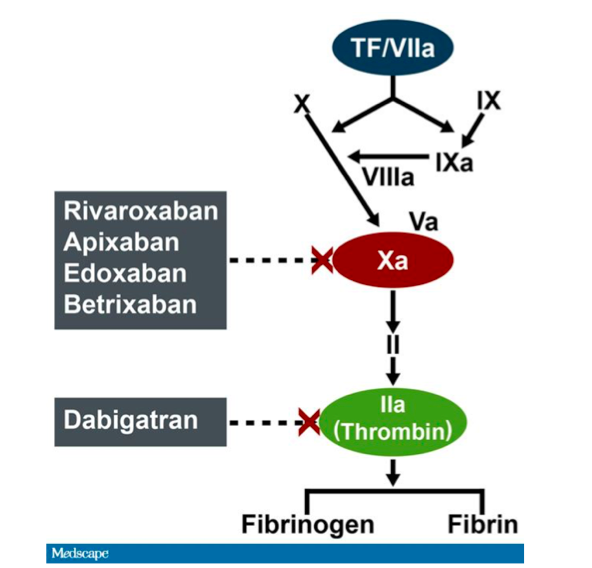
Patients enrolled in RE-VERSE AD were older. Secondary endpoints included hemostasis during the surgery or procedure. The primary endpoint was maximum reversal of dabigatran assessed using coagulation assays: diluted thrombin time and ecarin clotting time. Blood samples were taken at approximately 20 minutes, 1 hour, 2 hours, and 4 hours after the second infusion of idarucizumab. Two infusions of idarucizumab 2.5 mg were administered no more than 15 minutes apart. RE-VERSE AD enrolled 202 patients who required urgent surgery or a catheter-based procedure within 8 hours. Levy, MD, of Duke, presented greater detail on patients undergoing emergency surgery or an invasive procedure in the RE-VERSE AD study at the American Heart Association's Scientific Sessions held November 11 to 15, 2017, in Anaheim, CA. The trial examined the ability of idarucizumab, an antigen-binding fragment with a high affinity for dabigatran, to reverse the anticoagulant effect of dabigatran in patients with life-threatening bleeding or those requiring emergency surgery/invasive procedures. RE-VERSE AD was a multicenter, open-label prospective study that enrolled 503 adults taking dabigatran at 173 centers in 39 countries. Dabigatran, a direct thrombin inhibitor, is used for stroke prevention in nonvalvular atrial fibrillation (AF) and the prevention and treatment of venous thromboembolism (VTE).


 0 kommentar(er)
0 kommentar(er)
